FerriScan for Pharma
Fully Customized Imaging CRO Services
Resonance Health provides comprehensive clinical trial services for pharmaceutical companies using imaging end points in their Phase II, III, and IV clinical trials. A fully HIPAA, HITECH, and GDPR compliant web based infrastructure enables image data and results to be securely transferred from anywhere in the world to and from our core lab in Australia. Resonance Health has extensive experience and expertise in providing objective, reproducible, and quantitative imaging measurements and core laboratory services, and has provided these services for many clinical trials worldwide.
Resonance Health currently has 4 products with international regulatory clearances, including FerriScan, HepaFat-Scan, FerriSmart, and HepaFat-AI. In addition to core lab services, if clients require specialist reading services in other disciplines such as cardiology, Resonance Health is able to engage and manage fully audited and reputable third-party resources whenever necessary to provide a complete turnkey solution for clients seeking imaging CRO solutions.
Our Gold Standard MRI technology (FerriScan) for liver iron concentration measurement alongside our Cardiac T2* MRI service has become established in many international ‘Standards of Care’ for Thalassemia and we have over a decade of expertise in this clinical indication, providing a meticulous, standardized and quality assured approach to a comprehensive range of imaging core lab services for pharmaceutical partners, researchers, and clinicians.
Resonance Health partners with many key stakeholders and prestigious organisations in the global hemoglobinopathy and metabolic disease clinical and patient communities, adding further value to our research partners who leverage our expertise, networks and close collaborations.
Resonance Health’s expertise in the provision of imaging core lab services for pharmaceutical company clinical trials has been used in many multinational, multicenter studies over 15 years. Imaging biomarkers are used in clinical studies providing a safe, non-invasive alternative to invasive procedures such as liver biopsy. Imaging provides an ideal solution where repeat measurements are required over the life of the study.
Resonance Health has been involved in clinical studies where our non-invasive measurements have been used to assist in:
- The assessment of subject inclusion or exclusion;
- Supporting primary and secondary end-points demonstrating drug efficacy;
- Evaluating the safety profile of a new therapy;
- Decisions on dose modifications.
Our continually growing clinical network consists of primary and secondary care providers, key opinion leading clinicians and imaging centers worldwide.
Overview of Services
- Resonance Health produces products and services from an ISO 13485:2016 certified central core laboratory, delivering quantitative image analyses globally. The Company has five products with international regulatory clearances :
- FerriScan® R2-MRI– internationally recognised as the gold standard in liver iron concentration (LIC) measurement. FerriScan is cleared by the FDA, CE Mark, and TGA, plus companion diagnostic for deferasirox clearance from FDA.
- Cardiac T2* for Iron Assessment – offered as a dual analysis service with FerriScan to provide information regarding cardiac iron stores;
- HepaFat-Scan® – volumetric liver fat fraction (VLFF) for quantitative measurement of hepatic steatosis. Cleared by the FDA, CE Mark, and TGA;
- HepaFat-AI – automatically analyses MRI datasets to assess liver fat in patients. Reports a steatosis grading, proton density fat fraction (PDFF), and volumetric liver fat fraction (VLFF). Received 510(k) clearance from the US FDA, European CE marking, and approval from the Australian TGA;
- Bone Marrow R2-MRI for Iron Assessment – provides an estimation of iron levels in the bone marrow.
- FerriSmart – a trained neural network tool for the quantification of liver iron concentration. Clearance from FDA, CE Mark, TGA plus companion diagnostic for deferasirox clearance from FDA.
- Quantitative Image Analysis available globally in an investigational setting:
- Quantitative Iron Assessment in Other Organs – surrogate iron measurements (R2 / R2*) in other organs including pancreas, spleen, and kidney;
- Brain iron – several brain iron imaging protocols to quantify iron deposition in various regions of the brain such as leptomeninges, basal ganglia, etc;
- Pancreatic Fat Assessment– quantitative assessment of pancreatic fat;
- Visceral / Subcutaneous Fat and Organ Fat in Metabolic Disease – quantitative assessments of visceral fat, subcutaneous fat, epicardial fat;
- Fibrosis and Inflammation – a combination of MRI measures to assess liver fibrosis and inflammation;
- Liver Biopsy – Stereology Services– quantitative assessment of hepatic steatosis of digitised biopsies using stereology;
- Organ Volume Measurements – measurements of various organs such as the liver and spleen;
- Other – customised design protocols on an as required basis. Examples include protocols to assess tracer entry into cells (eg. gadolinium) to attempt to monitor drug delivery; novel cardiac imaging protocols; and many others.
- Scanner verification using phantoms (in accordance with the FDA Draft Guidance Clinical Trial Imaging Endpoints Process Standard, March 2015)
- Secure 21 CFR Part 11 compliant web-based infrastructure for image transfer and reporting
- Imaging trial design consultancy
- Core Laboratory Services including project and data management
The Resonance Health team includes scientists, engineers, researchers, regulatory affairs expertise, and quality management. Our specially trained physicists and engineers are experts in image analysis using our patented world leading technologies, and are supported by the wider research and development team at the forefront of imaging analysis development. Resonance Health also partners with many other prestigious organisations, including government research bodies, universities, international patient organisations, and centres of excellence to develop and provide world leading solutions to the clinical and pharmaceutical communities.
Standardisation
Resonance Health tightly controls standardisation of imaging data acquisition and processing. This is done using standardised acquisition protocols, the use of phantoms (sets of standards with known R2 or T2* values), and involvement from our team of physicists and engineers to liaise with sites at setup. Each MRI centre must submit phantom image data that meets the quality control requirements of Resonance Health for that product. Our team will liaise with each site to provide instructions for setup, troubleshooting, and assistance, and will confirm that the site is correctly set up for patient scanning (‘ verification’). Our team is highly experienced at liaising with and setting up sites remotely, with a 15 year history of doing so. Once our service team has determined that a scanner is correctly set up, trial subject scanning can begin. A patient participating in a clinical trial will be referred to a verified site for an MRI scan. The site will acquire data and upload the data to Resonance Health via our HIPAA, HITECH, and GDPR compliant ‘FAST’ system; a web-portal for the secure transmission of image data and patient results from anywhere in the world. Once received, the raw data is subject to stringent quality control checks, and if deemed suitable, is analysed. The results report is made available for download to authorised persons within a target turnaround time of two business days.
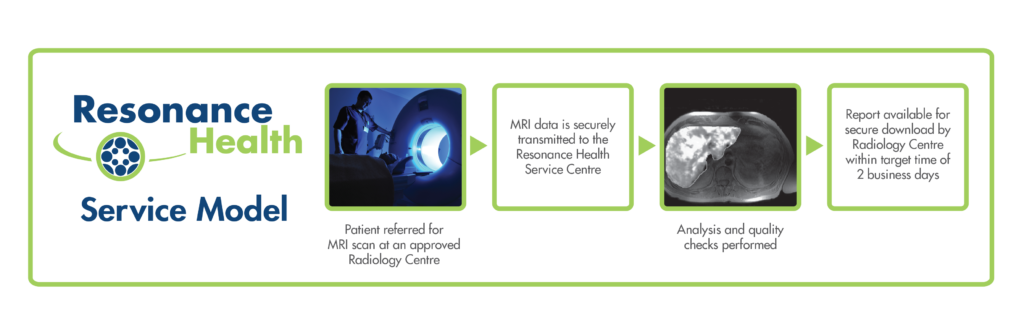
Our service model, utilising a central Service Centre with a team of experienced analysts, allows vigilant Quality Control and Quality Assurance measures to be performed for each and every analysis. As such, Resonance Health can assure the reliability and reproducibility of our results in accordance with our certified quality management system.
Regulatory Compliance - Data Integrity
Resonance Health has a company-wide commitment to quality management, and it is the responsibility of every employee, in every activity. Our regulatory clearances, together with our quality commitment and certification, provide our customers with the confidence they demand from a CRO service.
All aspects of our service provision are performed in accordance with our certified quality management system. From the recruitment and training of staff, to the receival of data and the quality control checking of results, documented procedures are followed and records retained.
Resonance Health complies with the following requirements:
- ISO 13485:2016 Medical Devices – Quality Management Systems;
- Food and Drug Administration (FDA) Code of Federal Regulations (CFR), Part 820 – Quality System Regulation (QSR);
- Compliance with GMP and GCP.
Resonance Health also utilises regulatory cleared medical devices to provide its analysis services, which are cleared in the following jurisdictions:
- Europe: all products have CE mark and are compliant with the European Medical Devices Regulation (MDR) 2017/745;
- USA: all products are FDA cleared (Class II medical devices);
- Australia: all products are listed on the ARTG and are compliant with the following requirements: Therapeutic Goods Act 1989 and the Therapeutic Goods (Medical Devices) Regulations 2002;
- New-Zealand: all products are registered within MedSafe and are compliant with the current Medicines Act 1981;
- Canada: all products are compliant with the current Canadian Medical Devices Regulations (SOR 98/182).
Data Security
Resonance Health has undertaken extensive measures to ensure the security of patient data.
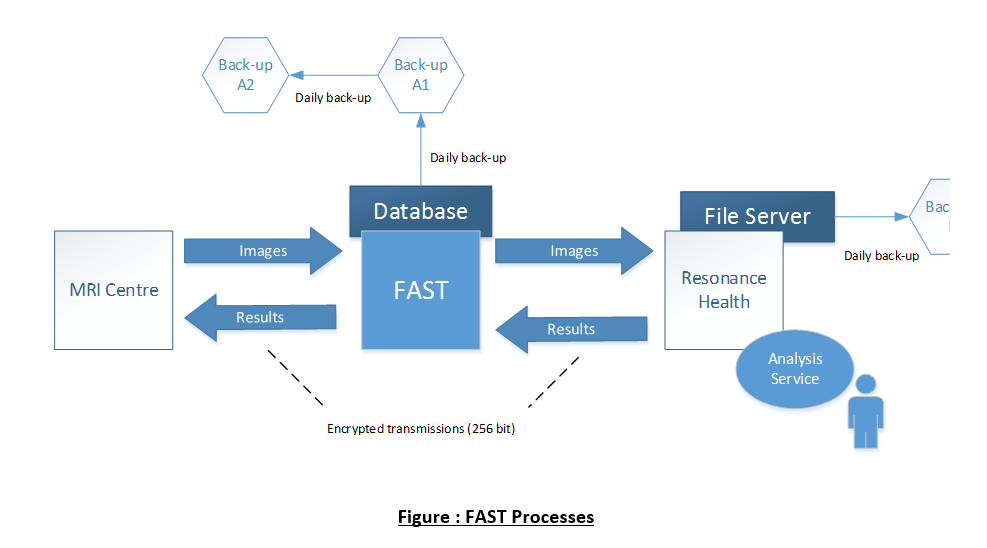
Resonance Health utilises its proprietary secure web-based system (FAST) for data upload, tracking, and result download. All data transfer to and from the FAST system is secure. Access to the FAST system is restricted by unique login IDs and passwords. FAST has an access control system based on unique user identification, which restricts Resonance Health staff to access PHI only where it is necessary for them to perform their role. Likewise, staff at the MRI Centre are only provided with access to the type of Patient Reports they require access to. For example, this may include all Patient Reports or only those associated with a particular clinical trial.
In conjunction with electronic data security and protection, Resonance Health also has comprehensive physical data protection. All documents which contain patient information are stored in access-controlled facility and only authorised staff may enter this area.
Resonance Health provides services and products that are compliant with United States HIPAA and HITECH Acts, the European General Data Protection Regulation (GDPR) (Regulation (EU) 2016/679), the Australian MTAA Code of Practice, AdvaMed Code of Practice, Eucomed Code of Ethics and the Australian Privacy Act.
The measures include data and internet security, staff training, de-identification of patient data, quality control procedures, and physical security systems within our Service Centre.
Project Management Services
- Establishment of study specific project management files;
- Establishment of precise communication plans with Client to ensure effective exchange of trial critical information by:
- Drafting of study-specific communication plans;
- Assessing and making recommendations to fully satisfy all communication needs;
- Providing timely reports to meet critical project timelines;
- Offering a range of reporting options and frequencies;
- Providing excellence in customer service;
- Development of complete imaging charters and manuals as required by client. With full CRO services, this includes management of external expert parties to develop and deliver imaging charters through Resonance for any specific imaging requirements of a client that are outside of Resonance’s in-house expertise. Examples include ECHO, DXA, chest x-rays, or any other type of imaging required by client. We work closely with several experienced imaging, cardiology, and radiology groups to provide these extra services as required;
- Imaging center management and training. This includes training on any third party developed imaging charters and requirements, as necessary;
- Resonance Health provides a central dedicated point of contact for reporting and issue escalation. Resonance provides a protocol for issue reporting, escalation, and resolution for the Client to ensure that the client is aware at all times of the relevant Resonance personnel for contact;
- Standardized data acquisition and analysis protocols that have proven fidelity in clinical use and trial settings over many years.
- Reporting of trial status, such as:
- Ongoing site and scanner setup and verification status;
- Data query trackers;
- Data reconciliation trackers;
- Data delivery times;
- Subject results;
- Subject status tracker (i.e scan due dates, scompletion dates, overdue scans);
- Reporting cycles as requested;
- Fully customizable study status reporting as requested, including but not limited to, the following examples: study status reports; patient summary reports, adverse events (AE) and serious adverse event (SAE) reports; safety monitoring reports.
- Enforcing protocol patient identifier anonymity, including data query communications where necessary to confirm or correct patient identifiers;
- Trial specific SOPs and detailed work instructions to ensure trial protocol requirements are met, and Resonance staff members are trained on specific requirements;
- Maintenance of electronic and hardcopy trial related records in dedicated secure locations;
- Dedicated internal auditing of the trial records and data management;
- Record Retention: Resonance will retain records for no less than fifteen (15) years from expiration or termination of an agreement for such services. Resonance will not dispose of any records without the prior consent from our client;
- Provision of trial specific documentation as required, including an MRI Center Manual. Protocol specific instructions can be added to the result reports if required. These electronic reports are designed and validated according to the client’s needs;
- Compliance with all trial related regulatory requirements (in addition to ISO 13485, FDA 21 CFR Part 820 and the EU MDD), including 21 CFR Part 11, HIPAA and HITECH Acts, ICH Guidelines, GDPR (General Data Protection Regulations 2016/679), and Safe Harbor provisions. Induction and annual refresher for Resonance Health staff training on these requirements as standard;
- Full support of regulatory authority audits relating to specific clinical validation trials;
- Full support for Client audit requirements, including desktop audits, conference calls and in-person inspections of the Resonance quality system and services;
- Full design control and validation of ancillary processes and systems required for service delivery for clinical trials and record retention requirements;
- Establishment of a data transfer specification and database;
- Reporting of results and data cleaning as required;
- Electronic imaging lodgement and tracking.