FerriScan for Clinicians
Why Choose FerriScan
FerriScan provides an accurate measurement of liver iron concentration (LIC) through a non-invasive, MRI-based technology. FerriScan was regulatory cleared by the FDA for the measurement of LIC in 2005. In January 2013 FerriScan gained an additional clearance from the FDA as a companion diagnostic to aid in the identification and monitoring of non-transfusion-dependent thalassemia patients receiving therapy with deferasirox. FerriScan is now internationally recongized as the gold standard in liver iron concentration (LIC) measurement.
FerriScan has originally been validated against liver biopsy on several makes and models of MR scanner in hundreds of patients, and has high sensitivity and specificity for measuring LIC. FerriScan results are clinically validated to be unaffected by inflammation, fibrosis or cirrhosis. To date, FerriScan has been used for over 50,000 patient measurements at hundreds of MR centers worldwide. FerriScan has also now been used in many multi-national, multi-site clinical trials by pharmaceutical companies.
Advantages of FerriScan over serum ferritin (SF)
- Other factors present in patients with iron overload such as infection, inflammation, fever, cancer, metabolic disease, or liver damage may result in significant elevation of serum ferritin concentrations in the absence of iron overload;
- SF has poor accuracy for measuring body iron loading in patients with thalassaemia major;
- SF levels in patients with thalassaemia intermedia are significantly lower than in patients with thalassaemia major despite them having comparable Liver Iron Concentration (LIC) levels (as determined by biopsy), suggesting that SF significantly underestimates iron loading in patients with thalassaemia Intermedia;
- Increased serum ferritin concentrations may indicate iron overload, but are not a quantitative measure of iron burden. Additionally, once SF becomes saturated, there is poor correlation between SF and iron levels;
- SF is an imprecise and potentially misleading parameter on which to base clinical management decision in patients with sickle cell disease (SCD);
- The relationship between total body iron stores and SF in patients with hereditary haemochromatosis is very weak;
- SF has significant limitations in assessment of iron burden in children with SCD, and liver iron assessment therefore is necessary for optimal management.
Advantages of FerriScan over liver biopsy
- Non-invasive, painless, no risk of bleeding or infection;
- Provides information about the distribution of iron in the liver;
- The size of the biopsy specimen only represents 1/50,000th of the total mass of the liver, therefore the sampling error is potentially large - the location where the biopsy is taken from will affect the result, and may not be representative of the entire liver;
- FerriScan® measures iron in a volume of liver that is thousands of times greater than liver biopsy and therefore has a much smaller sampling error.
Advantages of FerriScan over Liver T2* (MRI)
- There are a number of liver T2* techniques and these are not standardised. Non standardisation means that it is very difficult to compare results. See Graph below;
- The liver T2* techniques are not regulatory cleared;
- As a patient’s LIC starts to climb, the liver T2* techniques become more unreliable and eventually fail at higher iron levels. This can occur from 15 mg/g dry weight (depending on method of acquisition and analysis);
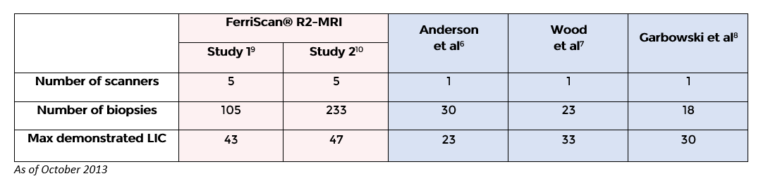
- The liver T2* methods do not report across the full range of iron loading seen in clinical practice. (Published liver T2* methods measured LICs up to 23.6, 27.67 and 32.98 mg/g dry tissue, respectively.);
- The liver T2* techniques can be influenced by the presence of liver fat and fibrosis;
- A review of the literature shows that, unlike FerriScan® R2-MRI, liver T2* methods generate data that are scanner and method dependent and hence are not sufficiently standardised to enable reliable liver iron concentration measurements using calibration curves published from other centres;
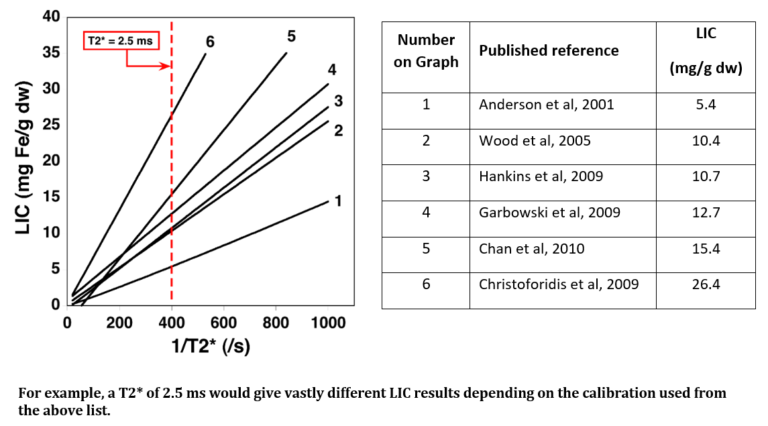
- Liver T2* to LIC conversion is dependent on a number of factors including various scanning profiles, various methods of analysis, and which calibration curve is used. The variance in several T2* techniques can be seen in the tables below. For the purposes of comparison, the LIC result derived from a calculated liver T2* value of 2.5ms will vary widely depending on which calibration curve has been used for conversion.
Comparison of Studies Conducted Using FerriScan and T2* MRI
Range of LIC Results Produced by Different T2* Calibrations
FerriScan Reimbursement
Canada
FerriScan is reimbursed in all Canadian provinces as an ‘Out-of-Country Medical Service’. The treating clinician must forward an application for each patient to the province’s Ministry of Health (MOH) for approval. Once approved, the MOH will grant a timeframe for the patient to have their FerriScan and/or Cardiac T2*.
To help with the application process, we’ve compiled a list of resources that may help you with your prior approval submissions:
Ontario
- Prior Approval for Full Payment of Insured Out-of-Country (OOC) Health Services – Rescheduled FerriScan
- Prior Approval for Full Payment of Insured Out-of-Country (OOC) Health Services – Annual FerriScan
- Prior Approval for Full Payment of Insured Out-of-Country (OOC) Health Services – Repeat FerriScan
- Prior Approval for Full Payment of Insured Out-of-Country (OOC) Health Services – Blank form
British Columbia
- Out-of-Country Health Services Funding Application – Prefilled (If it doesn’t open, try using Acrobat Reader)
- Out-of-Country Health Services Funding Application – Blank form (If it doesn’t open, try using Acrobat Reader)
Alberta
- Out-of-Country Health Services Claim Form – Prefilled (If it doesn’t open, try using Acrobat Reader)
- Out-of-Country Health Services Claim Form – Blank form (If it doesn’t open, try using Acrobat Reader)
- Out-of-Country Health Services Application Process – Information
Manitoba
- Pre-Approval FerriScan Information – FerriScan Info Pack (Word Doc)
- Manitoba Out-of-Province Medical Referrals – Information
Saskatchewan
- Pre-Approval FerriScan Information – FerriScan info Pack (Word Doc)
- Coverage for Services – Unavailable in Canada – Information
Quebec
- Pre-Approval FerriScan Information – FerriScan Info Pack (Word Doc)
- Medical services covered outside Quebec – Information
Nova Scotia
- Prior approval for out-of-country services – Information
Germany
Generally, in-patients receive full reimbursement for FerriScan and have no out-of-pocket expenses.
UK
FerriScan is reimbursed at public hospitals through the local NHS Trust system.
USA
FerriScan has been used in the United States since 2005, when FDA clearance for FerriScan was obtained. Since that time, over 13,000 FerriScans have been performed for US patients at a number of US hospitals and radiology facilities.
The cost of the MRI scan (or part of the cost) is usually covered by insurance companies (payers). Pre-authorisation is recommended, as is common for MRI testing. However, the cost associated with the FerriScan image analysis may not be covered, resulting in the hospital or patient having to cover this cost, together with any co-pay associated with the imaging.
FerriScan has now been deemed eligible for reimbursement by some US Insurance Companies:
- Health Net. Prior authorization is required to confirm the medical necessity of a FerriScan
- Kaiser Permanente SE Atlanta. Prior authorization is required to confirm the medical necessity of a FerriScan
- Medical Mutual of Ohio. No prior authorization is required
Supporting Documents
Click the link(s) below to access our resources. Need something else? Please contact our team at [email protected] for further information.